
会议概况
**CAR-TCR 2020 细胞与基因治疗创新峰会 **
10月15日-16日 | 上海浦东锦江汤臣洲际大酒店
大会官网:www.aprdl2020.com
350+ 来自政府监管机构、行业协会、科研院校、临床研究中心、大型跨国制药公司、创新型生物技术公司、CAR-T, TCR-T, CAR-NK, TIL, AAV-based Gene Therapy 细胞及基因治疗免疫疗法研发企业、新药研发设备平台、细胞和基因治疗服务公司、医药研发服务公司、临床试验研究机构、人工智能以及解决方案提供商、律所、投资以及咨询机构等药物研发领袖及高层代表共同参与并探讨:
- 细胞和基因治疗商业化和产业化之路
- CAR-T 生产工艺与建立中控及放行方法的考量
- 罕见病AAV基因治疗产品在国内的开发现状
- 开发新型细胞疗法产品治疗新冠肺炎COVID-19
- CAR-T/TCR-T/CAR-NK 细胞治疗产品的临床开发与最新进展
会议议程 _(以会议现场为准)
10月15日
Future Directions of Cell & Gene Therapy and Innovation in China
上午8:45-8:50
欢迎致辞
上午8:50-9:20
Engineering TCR-based Immunotherapy against Cancer and Viral Infection
Dr. Xin Lin
Professor and Chairman, School of Medicine, Department of Basic Medical Sciences, Tsinghua University Founder, China Immunotech ( Beijing ) Biotechnology Co., Ltd
上午9:20-9:50
Translating and Developing Novel Cell Therapy Product for Fighting against COVID-19
Dr. Yu Zhang
Senior Vice President and Chief Scientific Officer, VCANBIO Cell & Gene Engineering Co., Ltd
上午9:50-10:20
**Developing AAV-based Gene Therapy Products in China, First China International AAV-Based Gene Therapy in Leber’s ** Hereditary Optic Neuropathy
Dr. Alvin Luk
Chief Executive Officer, Neurophth Therapeutics
上午10:20-10:50
From Manufacturing to Clinical: How Cytiva Accelerate your Cell Therapy Process with Automated, Closed and Scalable Module System
Dr. Yulong Cheng
Product Manager Asia Cell Therapy, Cytiva
上午10:50-11:00
茶歇及展台访问
上午11:00-11:45
Panel Discussion: Strengthening Collaboration for Development & Commercialization CAR-T Cell Therapy in China
Moderator:
Dr. Yu Zhang
Senior Vice President and Chief Scientific Officer, VCANBIO Cell & Gene Engineering Co., Ltd
Panelists:
Mr. Tony Liu
Chief Executive Officer & Chief Financial Officer, Cellular Biomedicine Group
Mr. Xiaoliang Sang
Regional Commercial GM-Asia Cell & Gene Therapy, Cytiva
Dr. Ting He
Chief Executive Officer, ImmunoChina Pharmaceuticals
Dr. Lyu Lulu
Chief Executive Officer, Juventas Cell Therapy
Dr. Lei Zhang
Senior Director of Discovery, Fosun Kite Biotechnology
Dr. Jijun Yuan
Chief Executive Officer, S hanghai Genbase Biotechnology
上午11:45-12:15
The Demand of Novel Equipment for New Concept Cell Therapy Product
D r. Lei Zhang
Senior Director of R&D, Fosun Kite Biotechnology
上午12:15-12:45
The Investment and Venture Opportunities in Gene Therapy
Dr. Adam Zhao
Chairman and CEO,
Beijing Anlong Gene Medicine Technology
下午12:45-14:00
午宴及展台访问
下午14:00-14:30
Development Safe and Potent CD19 CAR-T Cell Therapy in China targeting Hematological Malignancies
Dr. Ting He Chief Executive Officer, ImmunoChina Pharmaceuticals
下午14:30-15:00
First-in-class Autologous CD7-CAR-T Cells Exhibited Promising Clinical Efficacy for Relapsed and Refractory T-lymphoblastic leukemia/lymphoma
Dr. Lin Yang Founder & Chief Executive Officer, PersonGen BioTherapeutics
下午15:00-15:30
Precision Immunotherapy: Development BCMA-Targeted CAR T-Cell Therapy for R/R Multiple Myeloma
Dr. Wen (Maxwell) Wang Chief Medical Officer, IASO Biotherapeutics
下午15:30-16:00
分布式个性化细胞药品智能生产
陈皓 先生
创始人, CEO/总工程师,
英诺维尔智能科技(苏州)有限公司
下午16:00-16:20
茶歇及展台访问
下午16:20-16:50
Surface Density of CAR Molecules Modulate the Kinetics of CAR-T Cells In Vivo
Dr. Jianqiang Li
Founder and Chief Scientific Officer, Hebei Senlang Biotech
下午16:50-17:20
CAR T Process Development and in Process and Batch Releasing Assay Development
Dr. Xinpo Jiang
Senior Director, Analytical/Process Validation/QC/QA, Legend Biotech
下午17:20-18:00
Panel Discussion: CAR T Process Development and Quality advancements in CAR-T cell manufacturing
Moderator:
Dr. Xinpo Jiang
Senior Director, Analytical/Process Validation/QC/QA, Legend Biotech
Panelists:
Dr. Lei Zhang
Senior Director of Discovery, Fosun Kite Biotechnology
Mr. James Chen
Chief Executive Officer, INNOVEL
Dr. Lin Yang
Founder & Chief Executive Officer, PersonGen BioTherapeutics
下午18:00-18:30
Novel Proceeding about Targeting CD 30 CAR-T Therapy for Lymphoma
Dr. Tongcun Zhang
Chief Executive Officer, Wuhan Bio-Raid Biotechnology
下午18:30
会议第二天结束
10月16日
Future Directions of Cell & Gene Therapy and Innovation in China
上午8:45-9:00
欢迎致辞
上午9:00-9:30
Precision TCR Redirected T Cell Immunity Treating Solid Tumour
**Dr. Yi Li
** President & Chief Scientific Officer, Guangdong Xiangxue Life Sciences
上午9:30-10:00
Solid Tumour CAR-T Barriers and Solutions
Dr. Enxiu Wang
Founder and Chief Executive Officer
Nanjing CART Medical Technology
上午10:00-10:30
The Latest Advancement in T-cell-based Cancer Immunotherapy
**Dr. Mingjun Wang
*Executive President, Shenzhen Institute for Innovation and Translational Medicine*
上午10:30-11:00
The Application and Advantages of Nano-bodies in Immuno-cellular Therapy Development
Dr. Jishuai Zhang
Chief Scientific Officer, The Pregene Biopharma Company
上午11:00-11:15
茶歇及展台访问
上午11:15-11:45
**Clinical Development of CAR T Cell Therapy & Next Generation CAR T Cells **
**Dr. Wei Wang
*Vice President of Clinical Development, CARsgen Therapeutics*
上午11:45-12:15
通过造血干细胞中的基因编辑技术治疗地中海贫血
吴宇轩 博士
研发副总裁, 上海邦耀生物科技有限公司
上午12:15-12:45
溶瘤病毒OH2的研究进展
刘滨磊 教授
董事长, 武汉滨会生物科技股份有限公司
下午12:45-14:00
午宴及展台访问
下午14:00-14:30
基于非病毒载体的CAR-T 细胞肿瘤治疗 孙艳 女士 副总裁, 细胞药物BU总经理, 上海细胞治疗集团
下午14:30-15:00
Next Generations of CAR-T Technologies –TruUCAR™ and FasTCAR™ Dr. Xinxin Wang Group Leader for TruUCAR Platform Development, Gracell Biotechnologies
下午15:00-15:30
What can Flow Cytometry Do for CAR-T
**王卉 博士
** 检验科负责人( 副院长级 ), 陆道培医疗集团
下午15:30-16:00
茶歇及展台访问
下午16:00-16:30
Mechanisms & Clinical Study of CAR-NK Cell Drugs to Treat Solid Tumors
Dr. Dayu Hu Chief Operating Officer, Asclepius Technology Company Group
下午16:30-17:00
DNT 细胞技术-从基础研究到临床治疗肿瘤细胞药的研发
杨黎明 博士 董事长兼首席执行官, 浙江瑞顺生物技术有限公司
下午17:0-17:30
Progress on TCR-T Development and Solid Tumor Treatment
Dr. Yi Zhu China Head, T-Cure Bioscience
下午17:45
会议第二天结束
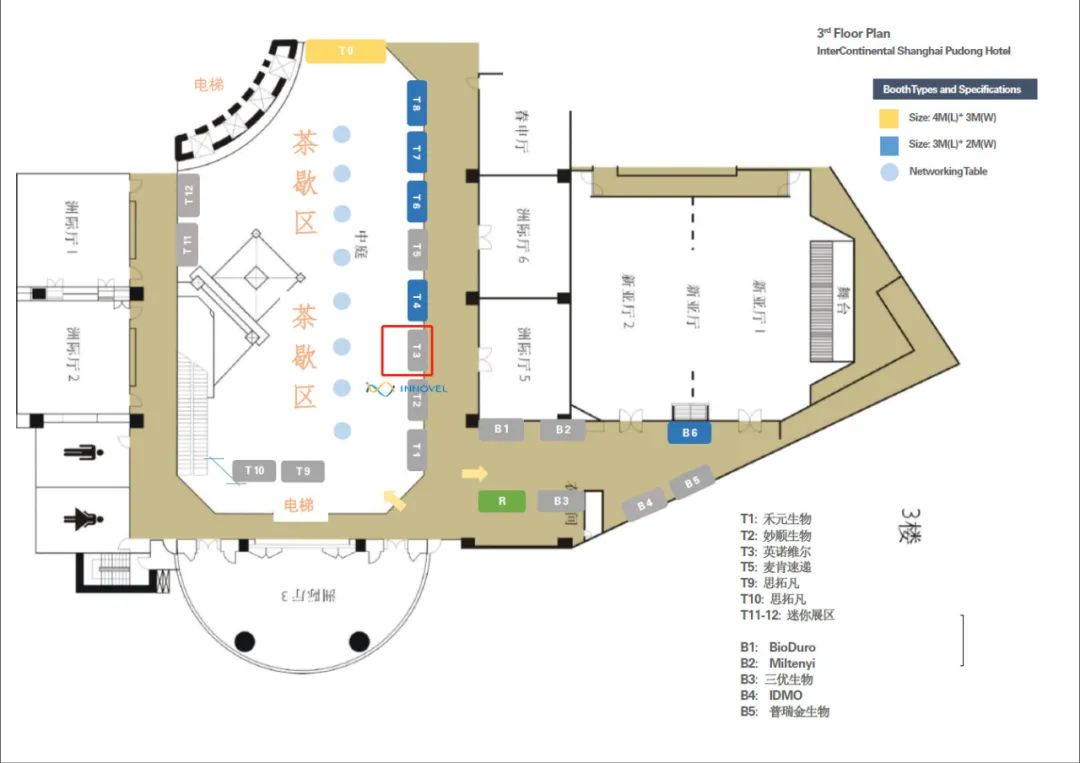
Innovel 展厅位置
参会指南
签到地点:上海锦江汤臣洲际酒店 三楼 新亚厅入口签到处
签到时间:10月15-16日07:50-17: 00
签到凭证:电子票条码(或票号)及名片或身份证
交通信息及停车:上海锦江汤臣洲际酒店(上海浦东新区张杨路777号)
公共交通:地铁2号线、4号线、6号线、9号线世纪大道站下步行8分钟
入住酒店的参会嘉宾可免费停车;未入住酒店的参会嘉宾可至签到处领取会议停车优惠券(5元/小时)
酒店住宿:
参会嘉宾入住会议酒店可享受协议价格800元/晚,如有需要请联系发送邮件david.xu@deliver-consulting.com 索取酒店预定表


