Lab Data Management System (LDMS)
End-to-end R&D Process and Data Management Platform
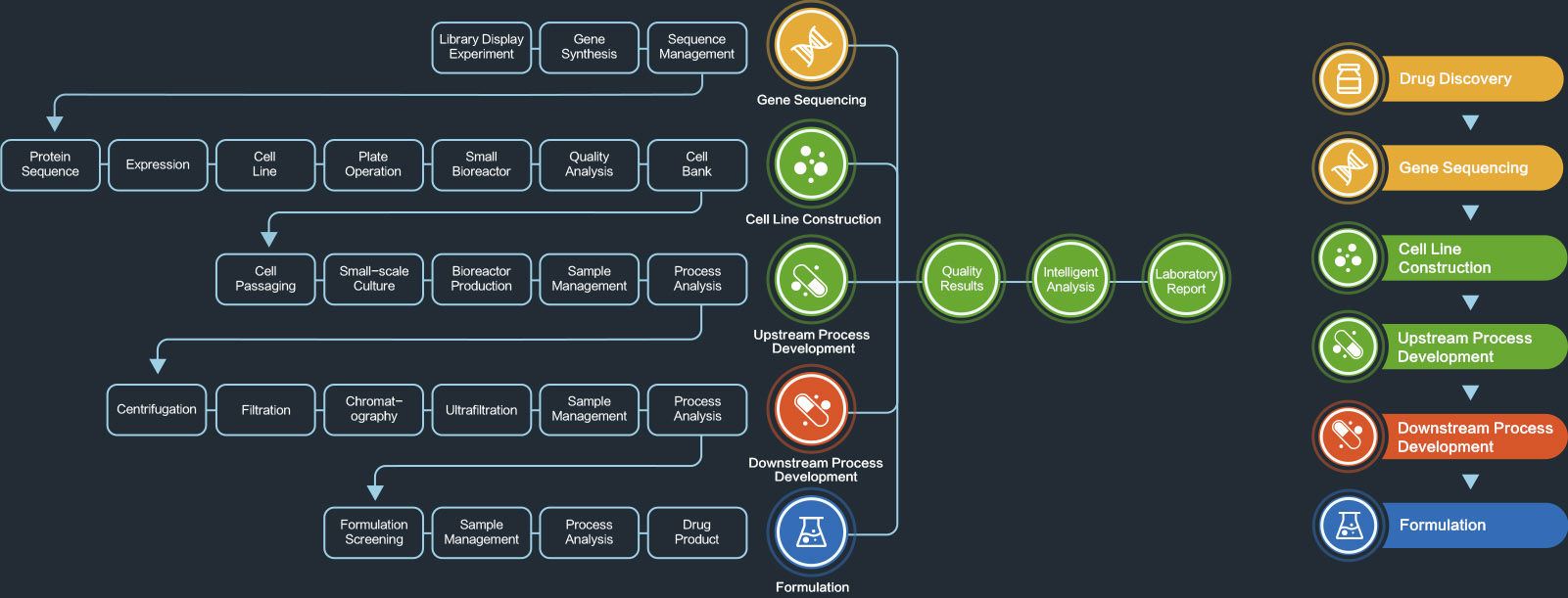
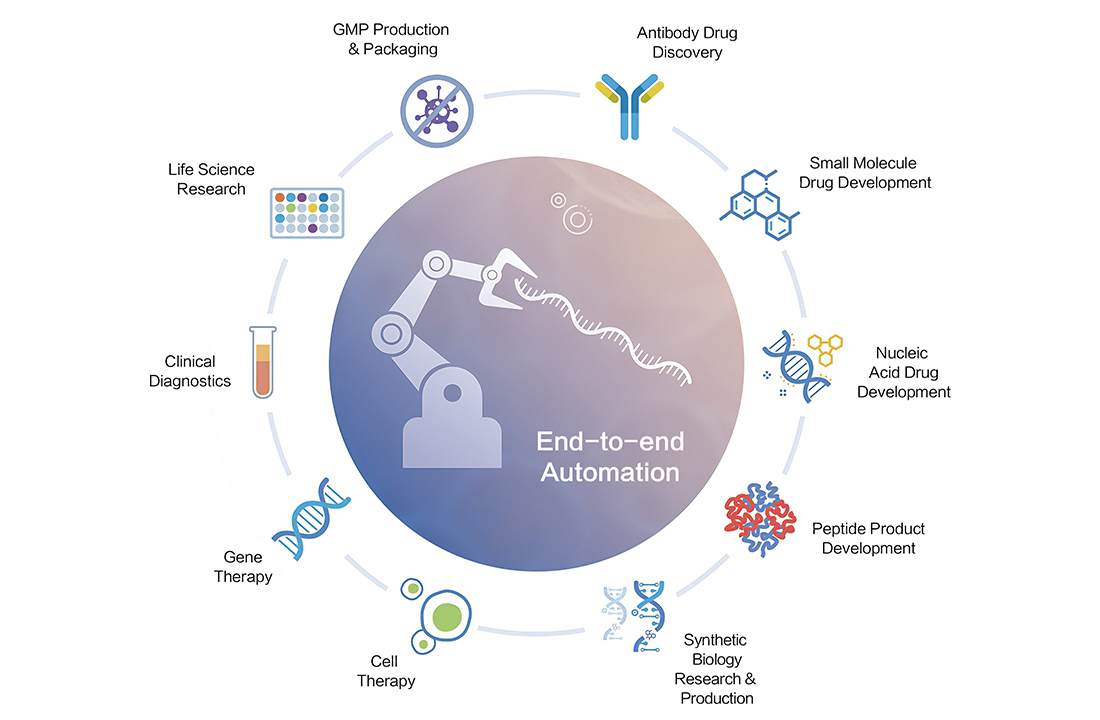
LDMS supports the end-to-end large molecule development and CMC workflow, including cell line development, upstream process development (UPD), downstream process development (DPD), formulation development (FD), process analytical support, and analytical development (AD).
The platform encompasses comprehensive experimental data and integrates all relevant peripheral data, such as data from bioreactors, downstream unit operations, or analytical instruments.
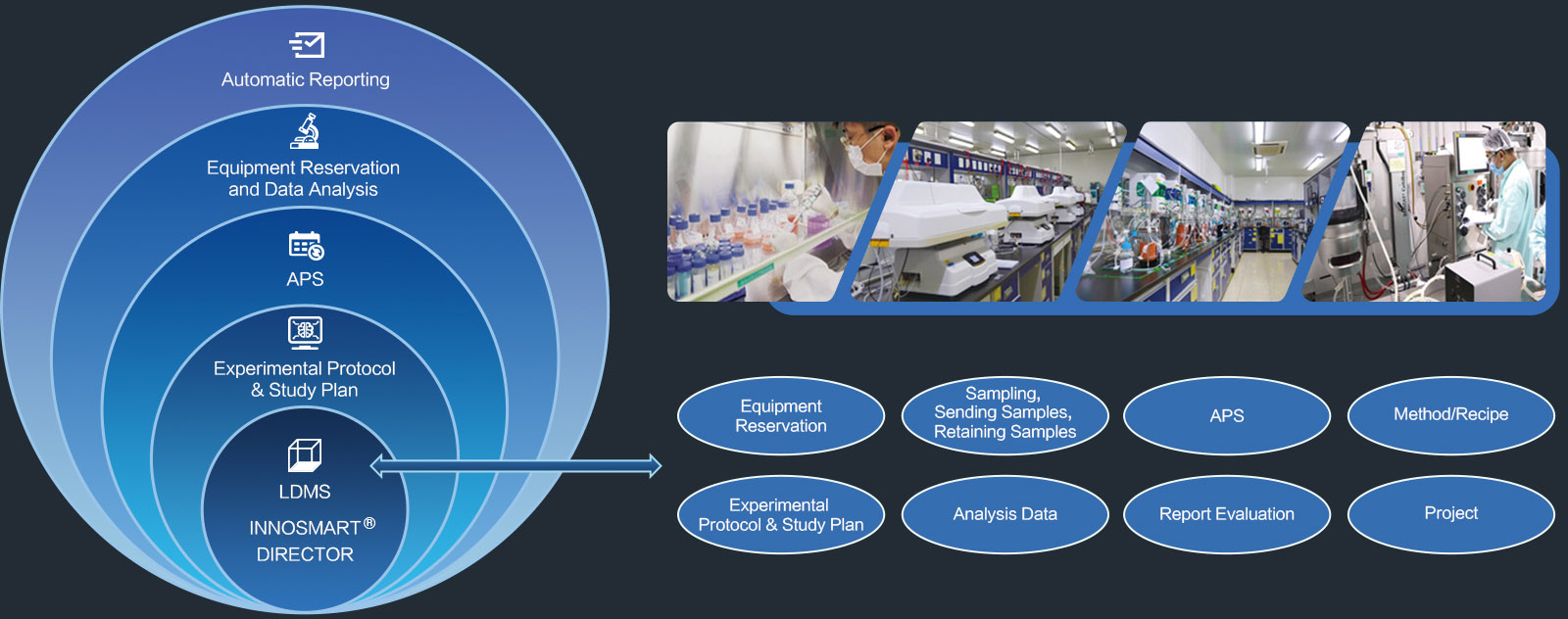
Lab Data Management System (LDMS)
INNOSMART ® DIRECTOR
Smart Lab Management System (SLMS)
- INNOSMART ® PROC
- Typical Process Scenario
- Cell Process Development
- Process Applications
- Automation Transformation
- Automatic Feeding Diagram
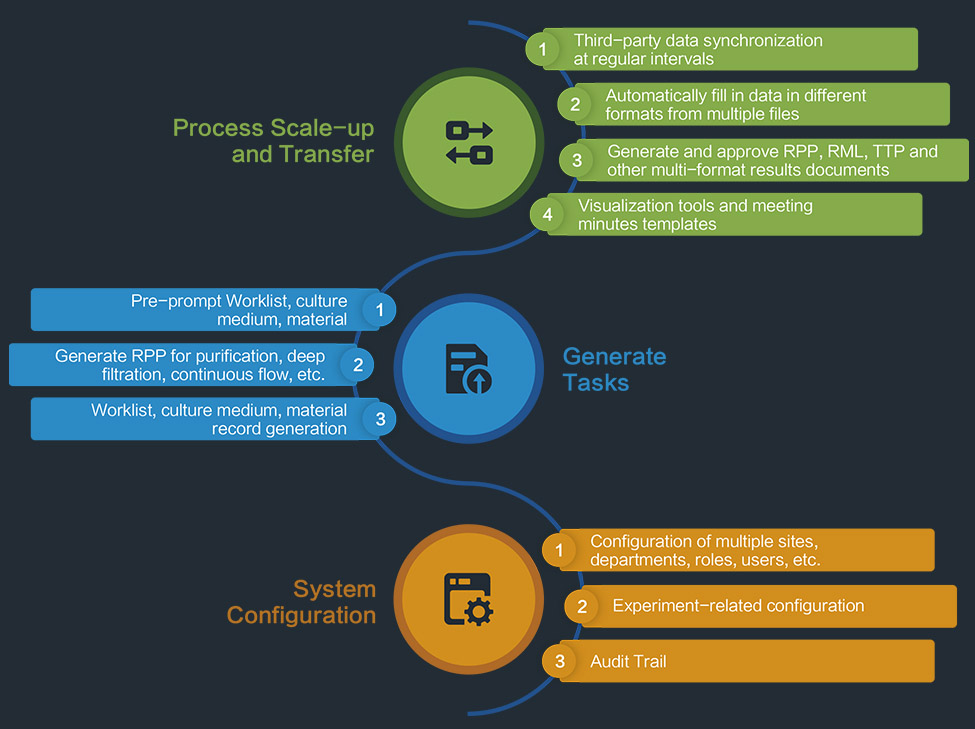
Technology Transfer System (TTS)
INNOSMART ® TTS
The system periodically synchronizes the locked technical parameter data after DOE, leveraging powerful and complex computing capabilities to analyze valid information from files and embed it into the forming templates.
A multi-level approval mechanism, combined with online discussions and annotations, enables a truly paperless workflow. It supports personalized dynamic data export in common file formats such as Word, Excel, PDF, and PPT.
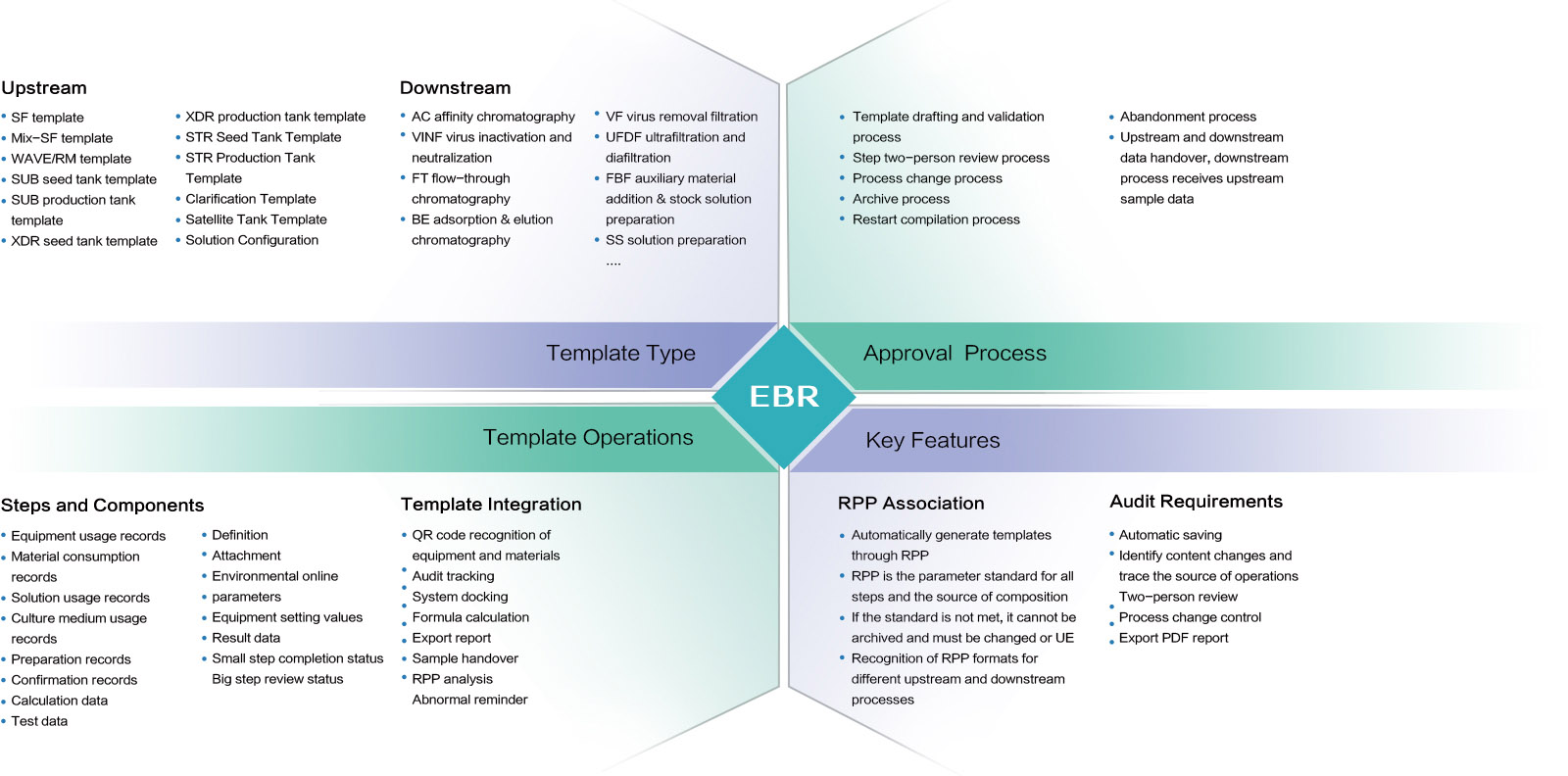
Electronic Batch Records (EBR)
INNOSMART ® EBR
The system dynamically analyzes the RPP parameter set to identify and generate template types and steps while embedding standard data as the basis for template parameters.
This significantly reduces the time required for manual experiment recording and minimizes computational complexity for researchers. Digital record-keeping effectively preserves operational history, providing strong support for future audit traceability.
Dashboard
Display a quick access entry for the current account’s records and a summary of pending tasks, making it easier for users to operate and manage the overall project.
RPP (Raw Process Parameters)
The system supports the import and real-time analysis of raw process parameters, enables autonomous template selection and generation, dynamically embeds standard data, and allows online data change approval.
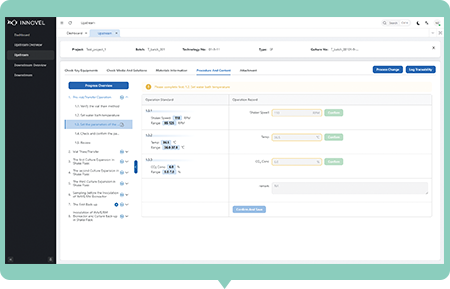
Electronic Records
Dynamically generate various experimental templates and procedures based on RPP, get equipment data with one click and calculate data correlations in real time, retain complete operation records, and meet the requirements of audit traceability.
Electronic Batch Records (EBR)
Scientific Data Management System (SDMS)
PI System and DeltaV Integration & IOT Internet of Things
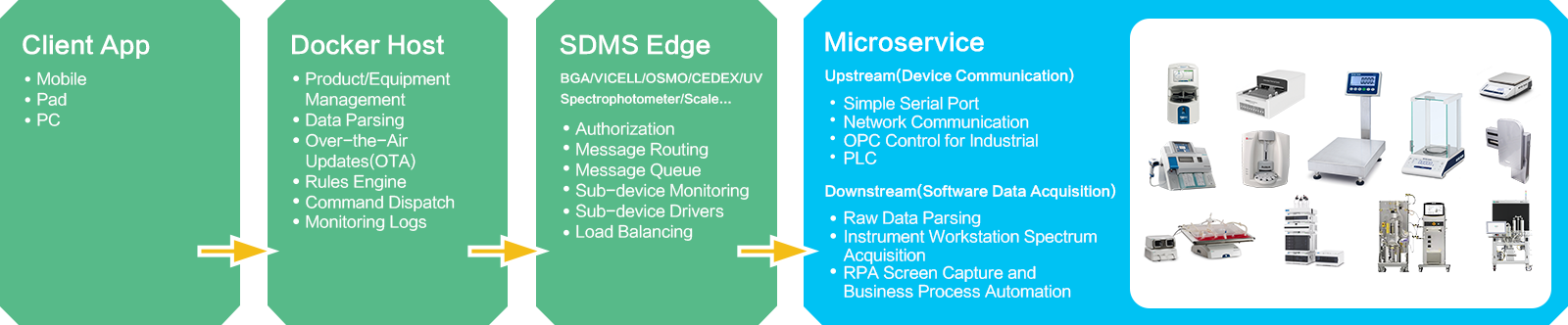
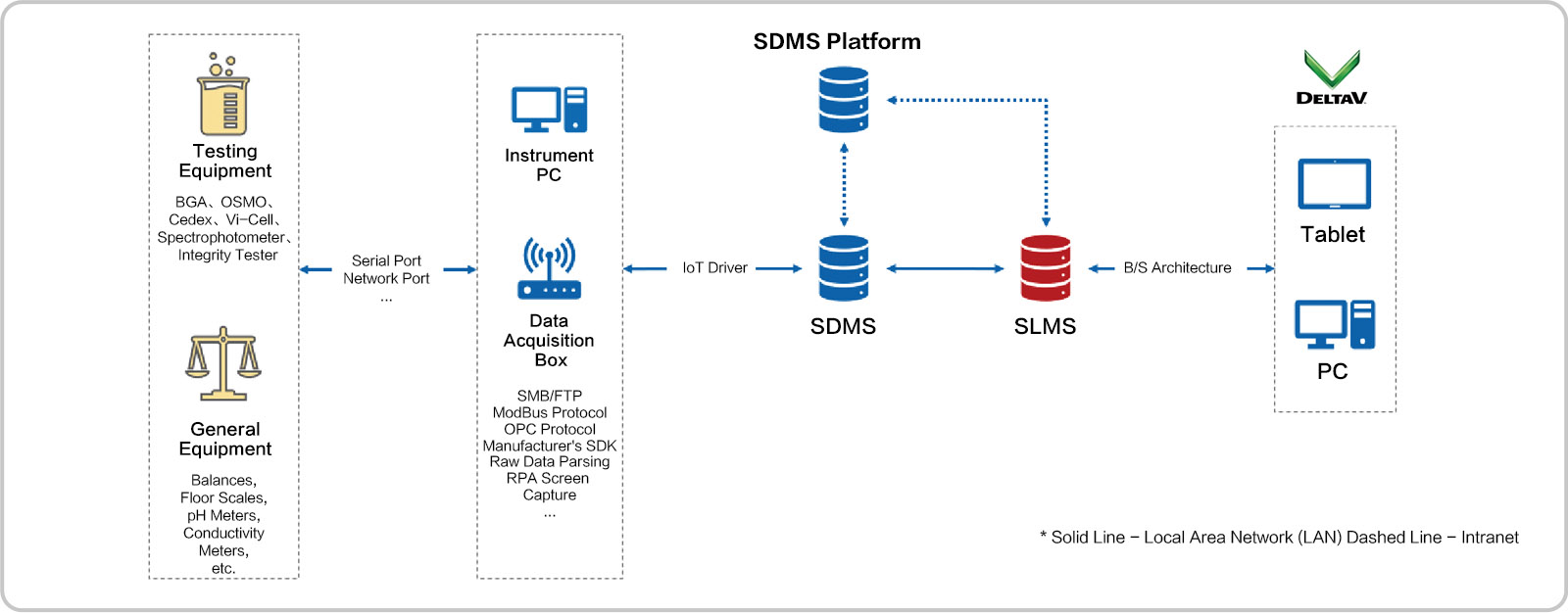
Scientific Data Management System (INNOSMART TRANSCEIVER) The instrument drivers of each Site/BU/Laboratory/Plant (INNOSMART OBSERVER) function as independent microservices. These drivers are managed on the SDMS Edge through an authorized RESTful API.
Whether in manual or automated applications, they interact with the SDMS platform via standard protocols, enabling the collection, control, debugging, and calibration of instruments. This setup facilitates easy replication and rapid deployment in the future.

Flexible interface-Rich experiences with over 200 analytics tools from third parties

Scientific Data Management System (SDMS)
Platform Architecture
Deployed in internal network of customer
Scientific Data Management System (SDMS)
An IOT Digital Suite
The suite comprises of the following:
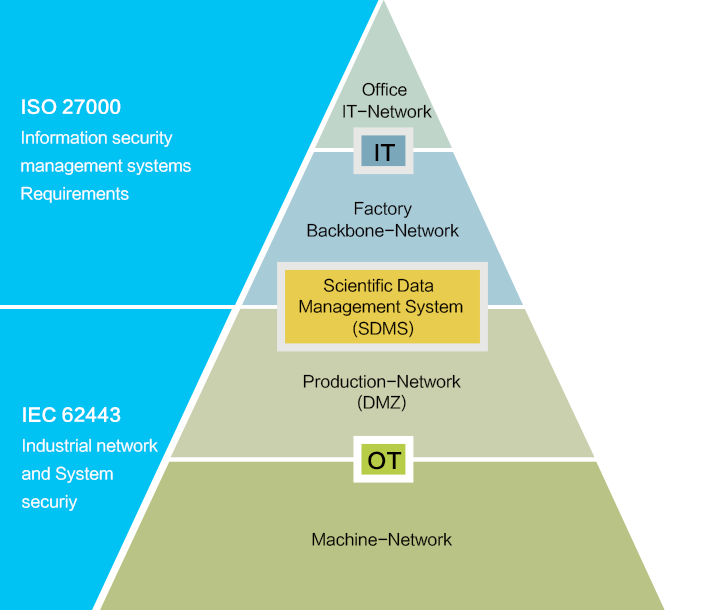
Enhances Cyber and Data Security
Compliance with Industry Standards:
Scientific Data Management System (SDMS)
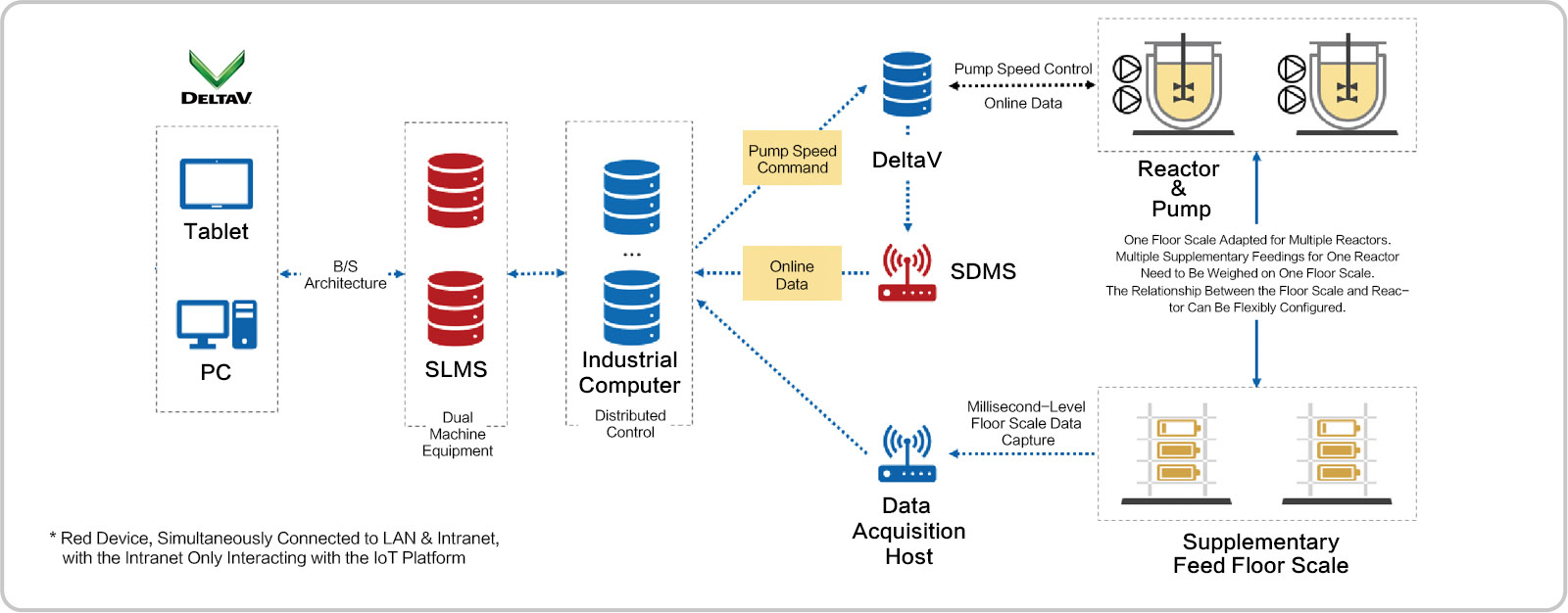
Automation Transformation
Scientific Data Management System (SDMS)
Automation Transformation